Mascharak Research Group
 |
Mascharak Research Group |
 |
Site Specific NO and CO Delivery to Biological Targets In recent years, metal nitrosyls like sodium nitroprusside have been used to control blood pressure and related hypertensive episodes. NO complexes that release NO upon illumination could also be employed as agents in Photodynamic Therapy. Since NO-induced cell death occurs via apoptotic pathways, site-specific NO-delivery could lead to destruction of malignant locales without intense immune response. In addition, the antibiotic effects of NO can be exploited to treat infection by bacterial pathogens. Since microorganisms seldom exhibit resistance toward NO, treatment of chronic infections by antibiotic-resistant variants of pathogens can be tackled with NO. During the past few years, Mascharak's group is involved in synthesizing designed metal nitrosyls that photorelease NO under very mild conditions (low intensity visible or UV light). Various chemical principles guide the design of such nitrosyls that deliver NO to biological targets under specific conditions. A variety of spectroscopic, magnetic and photochemical studies are performed to identify the factors responsible for the controlled release of NO from such nitrosyls upon illumination. Results of parallel theoretical studies are also utilized to elucidate the electronic origin of the NO photolability. In order to achieve site-specific delivery of NO, the NO-donors are entrapped in inert matrices such as polyurethane films, sol-gel patches, and porous inorganic materials. These materials can be selectively placed at biological targets and then conveniently triggered to release burst of NO upon illumination. Such light-triggered NO delivery from the nitrosyl-polymer composites have shown to cause apoptotic death of human malignant cells as well as eradication of loads of various gram-positive and negative bacteria and fungi. |
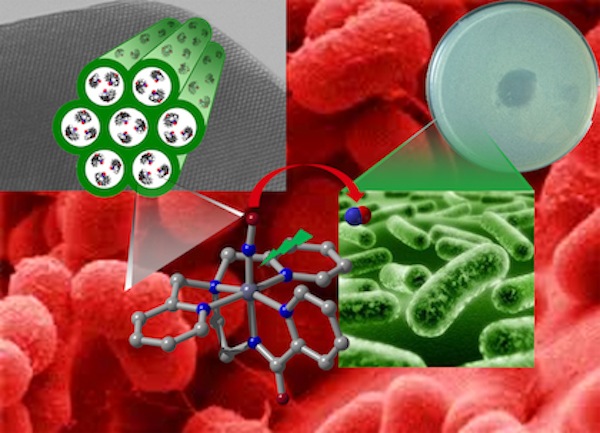 Photoreleased nitric oxide from a manganese nitrosyl entrapped within the pores of an aluminosilicate material (MCM-41) leads to eradication of Acinetobacter baumannii colonies on a skin and soft tissue infection model system Photoreleased nitric oxide from a manganese nitrosyl entrapped within the pores of an aluminosilicate material (MCM-41) leads to eradication of Acinetobacter baumannii colonies on a skin and soft tissue infection model system |
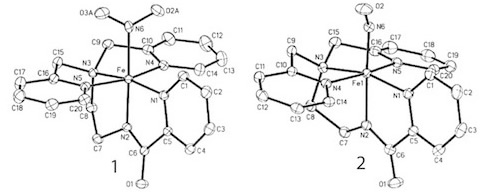
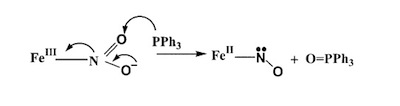
A recent synthetic analog of the active site of nitrile hydratase clearly establishes the fact that the oxygenated Cys-S centers are required for the observed NO photolability. |
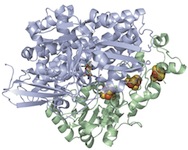
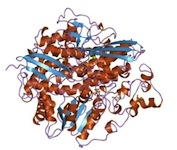
Modeling of the Active Sites of Metalloenzymes Another project in Mascharak’s group is in the area of modeling active sites of enzymes that contain transition metal ions. This research involves syntheses of metal complexes/clusters with biologically relevant designed ligands that mimic various metalloenzymes in their structural, spectroscopic and catalytic behaviors. The ultimate goal is to elucidate the mechanism(s) of the complex biological transformations occurring at the metal-containing active sites. At this time, two enzymes are under study. The first one, nitrile hydratase (NHase), is involved in microbial assimilation of organic nitriles. The active site of this enzyme contains either a low-spin Fe(III) or non-corrin Co(III) center. The second enzyme is acetyl Co-A synthase/CO dehydrogenase (ACS/CODH), an enzyme involved in CO2-fixation by various anaerobic chemotrophs. The so-called A-cluster of ACS/CODH contains a Ni-Ni-Fe4S4unit. Both these active sites have very unusual coordination structures. The metal centers are attached to the peptide backbone via carboxamido nitrogens and Cys-sulfurs. In addition, two of the three Cys-S centers of NHase are post-translationally modified to Cys-sulfenato (-SO-) and -sulfinato (-SO2-) groups. During the past few years, Mascharak's group has synthesized and structurally characterized series of designed metal complexes that resemble such active sites very closely and studied their reactivities to establish the mechanism of nitrile hydrolysis and acetyl group formation from CO and CH3 at the metal sites. Several of the model complexes have been used to perform analogous chemical transformations under very different conditions. Syntheses of novel bio-inspired catalysts on the basis of these studies are in progress. |
Oxygen Transfer using Green Reagents Another research project in Mascharak's group is related to the area of new "Green Chemistry". For some time, this group has been studying oxidation of various organic substrates (including alkanes and alkenes) by non-heme iron and cobalt complexes in conjunction with O2, H2O2 and ROOH. The goal of this project is to synthesize catalysts that operate under mild conditions (less energy requirements) and utilize safer reagents (like O2) for oxidations. |
 |
 |
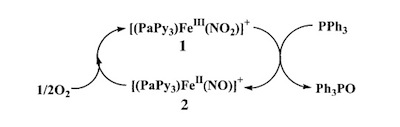 |